NSAA Guides
NSAA Section 2 - Your Definitive Guide to Advanced NSAA
Written by: Matt Amalfitano-Stroud
Please be aware that, as of 2024, the Natural Sciences Admissions Assessment (NSAA) is no longer being used by the University of Cambridge. Cambridge applicants for Natural Sciences, Engineering, Veterinary Medicine and Chemical Engineering & Biotechnology will be required to sit the Engineering and Science Admissions Test (ESAT).
Once you’ve completed Section 1 of the NSAA, it’s only natural that Section 2 will come next! This section of the exam will test your knowledge of Section 1 further while requiring you to use advanced science to answer more complex questions. The structure isn’t drastically different from what you’ve already seen, but the challenge will certainly be a step up. This guide will take you through what you need to know to get through Section 2 with confidence.
1/5
WHAT IS THE NSAA?
In case you’ve forgotten, or don’t already know, we’ll first quickly run through the basics of the NSAA to refresh your knowledge.

The Natural Sciences Admissions Assessment (NSAA) is the exam that must be taken by all applicants of Natural Sciences and Veterinary Medicine at Cambridge University. The NSAA is a pre-interview assessment and will be sat on October 18th 2023, which is a few weeks earlier than previous years.
Cambridge uses the NSAA to get an understanding of their applicants’ mathematical and scientific knowledge and abilities. These are key skills needed to succeed in either course so applicants must be able to show an aptitude for the subject, which the NSAA helps to determine.
How does the NSAA Work?
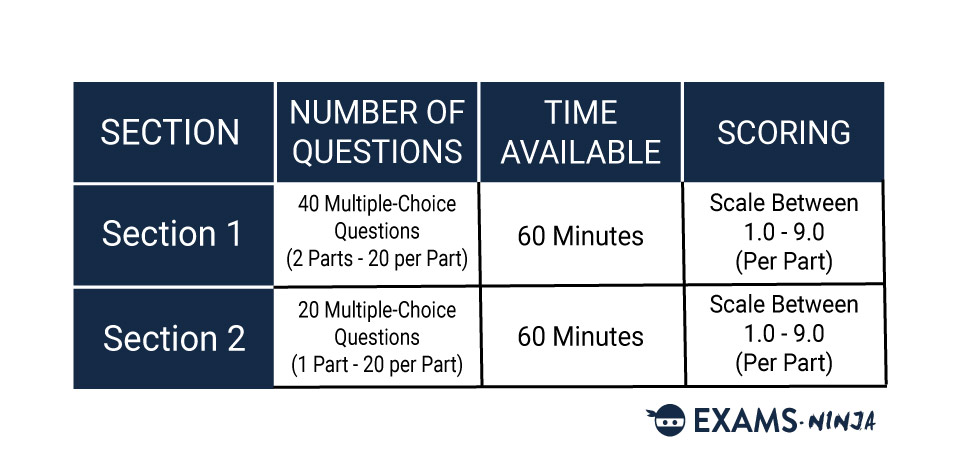
The NSAA consists of two separate Sections split between two papers, Section 1 and Section 2.
Section 1 will first see you solving mathematical problems followed by 20 questions relating to one of the three core sciences. You’ll only need to answer one set of questions out of the three.
Section 2 will also see you choosing one out of the three core sciences to answer questions on. These questions will combine knowledge of advanced science and advanced mathematics alongside the knowledge you use during Section 1.
Each collection of questions is referred to as a “part”, and you’ll be completing three of these throughout the whole of the NSAA, two in Section 1 and one in Section B. Each part in Sections 1 and 2 will have 20 questions to answer. You’ll have 60 minutes to complete each section but bear in mind that time from one section cannot be brought over to the other.
How is the NSAA Scored?
Scoring in the NSAA isn’t too complicated as all the questions are multiple choice. Each part of the NSAA is given a score on a scale between 1.0 and 9.0. This score can only be increased by answering questions correctly, meaning there’s no penalty for incorrect answers. Therefore, applicants should always aim to answer every question in the exam!
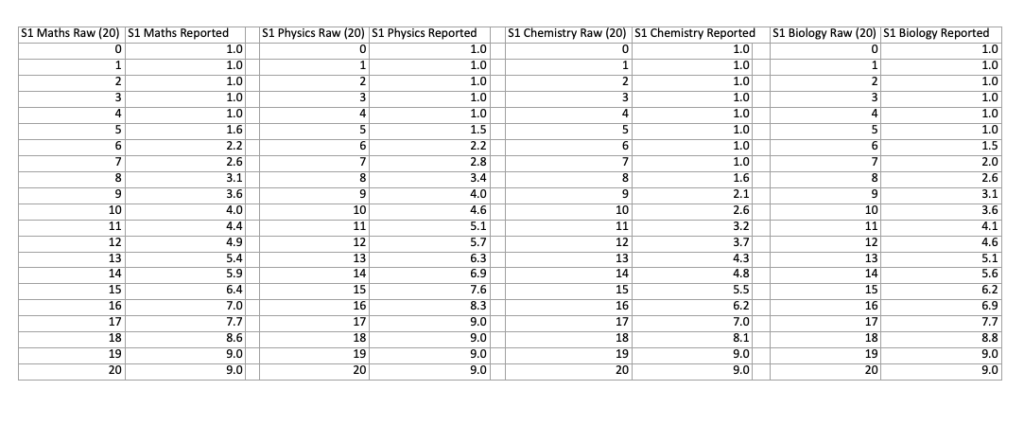
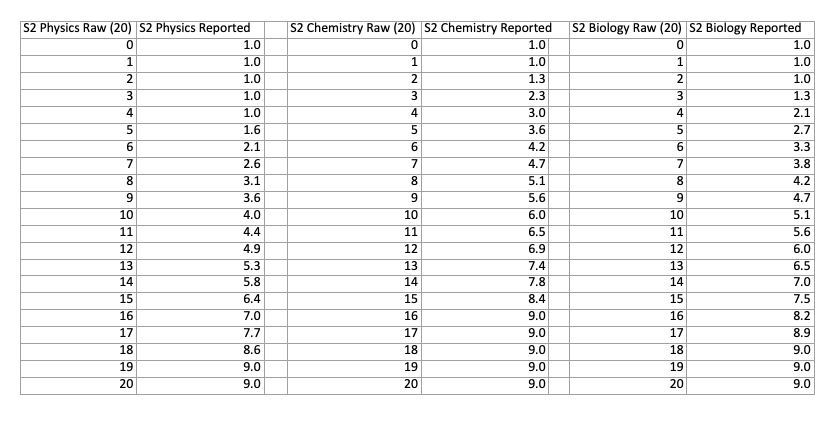
The conversions from marks to score are different for each part of the exam. You can compare the differences with the two charts below, which show the conversions for each part of both sections:
NSAA Section 1 Score Conversion
NSAA Section 2 Score Conversion
Note that the NSAA doesn’t have a unified passing or failing score. However, each college at Cambridge will have its own scoring standards, some of which are more strict than others.
The equipment you’ll need to bring with you for the exam is simple, just a soft pencil and an eraser. Calculators, formula books and other aids are not permitted for either section of the exam, although Section 2 will provide you with a full periodic table.
To learn more about the NSAA, take a look at our Definitive NSAA Guide.
The NSAA is a big undertaking, don’t do it alone!
Exams.Ninja will guide you through every step of your NSAA preparation, from revising the basics all the way through to perfecting your exam techniques. Check out the NSAA Preparation Platform to find out how you can get yourself ready for the big day!
2/5
WHAT TO EXPECT FROM THE NSAA SECTION 2
The two sections of the NSAA aren’t too different from each other, but it’s still important to know what changes to expect in Section 2, so you aren’t facing something unexpected!
By now, you should hopefully know that Section 1 has 40 questions to answer. This is the most noticeable difference between Section 1 and Section 2, as Section 2 will see you answering just 20 multiple-choice questions during the hour time limit.
That’s a big difference, isn’t it? Don’t mistake this for Section 2 being easier, however, as the questions you’ll need to answer require a combined knowledge of advanced mathematics and science, covering topics much more complicated than anything found in Section 1. While you will still need your basic skills at the ready, Section 2 is generally going to require a much more in-depth knowledge of your chosen field.
One other key difference is the context behind the questions. Section 1 has fairly basic questions that get straight to the point, while Section 2 relies much more on using contextual elements when finding the correct answer.
Exams.Ninja Tip
The most important thing anyone can do to solve a context heavy problem is to read the question thoroughly. Read it as many times as you need to make sure that you fully understand what is being asked of you.
Missing any details, no matter how small they may seem, could cause your whole solution to fall apart!
While Section 1 saw you answering two parts, including the mandatory mathematics part, Section 2 will only require you to answer one part. You will once again have to choose one of these three options to answer:
The 20 questions for each part will test you on a large variety of disciplines from the science you have chosen. You won’t have any indication of what questions will be asked before you receive the paper, so you’ll need to be sure you have revised as much as you can in order to confidently answer every question that you get.
Remember, you only need to answer the questions from one part! Answering any additional questions will not help increase your score and could potentially put your application at risk.
Let’s have a look at what a typical page looks like for each part of Section 2.
Each page of the paper is dedicated to a single question, providing you with plenty of extra space for working out. It’s vital that you use this space as no extra paper is permitted in the exam and the answer sheet is reserved solely for your final answer.
3/5
WHAT DO I NEED TO KNOW FOR SECTION 2?
Section 1 of the NSAA will have tested you on entry-level knowledge but Section 2 will require you to know advanced information and techniques. Let’s take a look at everything that you’re going to want to revise for the test.
All of the knowledge and techniques that you will have used in Section 1 will be required for questions within Section 2, but you are also going to need to know your advanced Mathematics, Physics and Chemistry. Biology does not have any advanced topics to revise, but this does not mean the questions will be any easier.
Firstly, let’s recap the basics for Mathematics, Physics and Chemistry that you’ll use in Section 1:
Mathematics
- Numeracy Skills
- Units
- Ratio and Proportion
- Geometry
- Statistics
- Probability
Physics
- Electricity
- Magnetism
- Mechanics
- Thermal Physics
- Matter
- Waves
- Radioactivity
Chemistry
- Atomic Structure
- The Periodic Table
- Chemical Reactions
- Quantitative Chemistry
- Group Chemistry
- Acids, Bases and Salts
- Rates of Reactions
- Carbon/Organic Chemistry
- Metals
- Chemical Tests
Biology
- Cells
- Movement Across Membranes
- Cell Division and Sex Determination
- Inheritance
- DNA
- Gene Technologies
- Variation
- Enzymes
- Animal Physiology
For a more in-depth rundown of each of these, check out our Section 1 Guide.
Now it’s time to get into the advanced stuff! Let’s start with Advanced Mathematics:
Advanced Mathematics
Although Section 2 does not feature a dedicated part to Mathematics, all of these disciplines will be used within questions for the three available parts. Here’s what you’ll need to know:
- Algebra and Functions - You’ll need to know about the laws of indices, use/manipulation of surds, quadratic functions, simultaneous equations, many-to-one mapping and algebraic manipulation of polynomials.
- Sequences and Series - You should understand arithmetic series, binomial expansion and the sum of a finite geometric series and to infinity for a convergent geometric series.
- Two Dimensional Coordinate Geometry - You need to be able to answer questions with straight-line equations (y – y1 = m(x – x1) and ax + by + c = 0), coordinate geometry of circles and various circle properties, including point tangents, semicircle angles and opposite angles.
- Trigonometry - You should have knowledge of the sine, cosine and tangent rules/functions, radian measure and simple trigonometric equations.
- Exponentials and Logarithms - You should know about y=ax and its graph, the laws of logarithms, and solutions to equations of the form ax=b.
- Differentiation - You’ll need to know the applications of differentiation to gradients, tangents, normals, stationary points, increasing functions and decreasing functions, as well as differentiation for xn and the derivative of f(x).
- Integration - Be sure to revise definite integration, finding/combing definite and indefinite integrals, approximation of area, differential equations and the Fundamental Theorem of Calculus.
- Graphs of functions - You should know graphs of common functions, the effects of simple transformations, altering values, geometric interpretation of algebraic equations, use of differentiation to determine graph shapes, use of algebraic techniques to determine intersections.
Want to know more about these topics? Our NSAA Mathematics Guide covers everything you need to know!
Part X - Advanced Physics
The first of the three parts, Advanced Physics will be testing your knowledge on the following:
- Forces and Equilibrium - You will need to understand the nature of scalars and vectors, components and resultants of vectors, moments, use of normal and frictional components of contact forces, application of centre of gravity and problem solving with the equilibrium of rigid bodies under coplanar forces.
- Kinematics - You should know about graphical methods involving distance, displacement, speed and velocity. You should also understand graphic representations of one-dimensional motion, equations of motion, and application of the equation “power = force x velocity”.
- Newton’s Laws - You need to be able to apply Newton’s Laws to linear motion, model a body moving vertically and solve problems with two connected bodies or with projectile motion.
- Momentum - You should understand the definition of linear momentum, the principles of momentum conservation, the relation of conservation of momentum to Newton’s Law and the application of impulse of force.
- Energy - You should revise the concepts of gravitational potential energy and kinetic energy, as well as the equations “power = rate of work = rate of energy transfer” and “efficiency = (useful energy transfer/total energy input) x 100%".
- Materials - You need to be able to apply the equations “density = mass/volume”, “pressure = normal force/area” and "young modulus = stress/strain". You’ll also need knowledge of tensile/compressive deformation, the behaviour of springs, application of Hooke’s law, and elastic/plastic deformation.
- Waves - Additional knowledge for this subject include wave motion, comparison of transverse and longitudinal waves, the principle of superposition, formulation of stationary waves, nodes/antinodes, reflection/refraction of waves, total internal reflection, critical angle and the equations “frequency = 1/period” and “speed = frequency x wavelength”.
- Electricity - You’ll need to understand Ohm’s law, Kirchhoff’s law, interpretation of V-I characteristics of an ohmic resistor, behaviour of LDRs/NTC thermistors, standard circuit symbols, circuit diagram interpretation, electromotive force, combined resistance, potential divider circuit and the following equations; “charge = current x time”, “potential difference = work done/charge”, “V = IR” and “resistivity = resistance x (cross-sectional area/length)”.
Part Y - Advanced Chemistry
Advanced Chemistry covers a lot of the same broad topics as before, but the questions will be much more demanding than Section 1. Here’s what you can expect to find:
- Atomic Structure - You’ll need to remember about time-of-flight (ToF) mass spectrometers, first ionisation energy, the electron configuration of atoms/corresponding ions and how first and successive ionisation energies relate to electron shells and sub-shells.
- Bonding and Structure - You should understand permanent/induced dipole, electronegativity, polar bonds and predicting shapes of simple molecules and ions.
- Energetics - You’ll need to be able to calculate enthalpy changes using Hess’s law and quantitatively use the terms “standard enthalpy changes of reaction”, “formation” and “combustion”.
- Kinetics - You should be able to draw and interpret Maxwell-Boltzmann distribution curves relating to two different temperatures.
- Equilibria - You need to know how to use Le Chatelier’s principle in deduction of qualitative effect on temperature and be able to construct an expression for the equilibrium constant “K”.
- Redox - You must know how to apply rules of assigning oxidation state to atoms in elements and deduce/combine half-equations of redox reactions. You should also understand oxidation and reduction in terms of electron transfer and oxidation state changes.
- Inorganic Chemistry and the Periodic Table - Be sure to revise how to describe reactions of chlorine with water/cold aqueous NaOH, the reactions of NaX with concentrated sulphuric acid and the organisation of elements in the periodic table. You should also revise the application of chemicals properties to Group 2 elements and their compounds.
- Organic Chemistry - You’ll need to understand structural isomers (including formulae of isomers), stereoisomers, reaction mechanisms, intermediate carbonations, single/double bonds, bond polarity, bond enthalpy, organic synthesis, chemical tests and organic compound structures with ion peaks and fragmentation peaks.
Part Z - Advanced Biology
New for 2022, the Advanced Biology portion of the NSAA covers many of the same topics as Part D, but now covers more complex concepts and question types. If you’re planning on taking on this brand new part, you’ll need to revise the following:
- Cell Structure - Following on from Standard Biology, you're going to need to dive deeper into Cell Structure, including uses and limitation of microscopes, the equation for magnification, the structure and function of components found within eukaryotic/prokaryotic cells and the interpretation of various visualisations of prokaryotic and eukaryotic cells.
- Biological Membranes - You should have a good understanding of the science behind membranes, including their roles, components, and processes. These include facilitated diffusion, osmosis and active transport. Also consider the effects of temperature on membranes.
- Cell Division and Organisation - Here, you will need to understand the major process used by plant and animal cells during interphase, mitosis and cytokinesis. Also be sure to revise meiosis, including its various stages.
- Biological Molecules - There's a lot to look at in this topic, so be sure to dedicate plenty of time to it! Assumed knowledge here includes monomers/polymers, carbohydrates, protiens, lipids and water. You should also know how to interpret results from various experiment types, which will likely come up in paper.
- Nucleotides and Nucleic Acids - Here, you will need to revise the general structure of nucleotides, comparisons of DNA and RNA structure as polynucleotides, semi-conservative DNA replication, the structure of adenosine triphosphate and the processes of transcription and translation within RNA.
- Enzymes - Most of what you need to know was covered within Standard Biology, but here you will also need to understand enzymes in the context of the lock-and-key hypothesis/induced-fit hypothesis. Plus you will need to understand the roles of intracellular/extracellular enzymes and enzyme catalysed reactions.
- Animal Physiology - This one's another big topic, as you will need to know about the mammalian gaseous exchange system (including its components), closed double circulatory systems, the equation for cardiac output (= heart rate x stroke volume), oxygen dissociation curves, heart changes caused by systole and diastole, the cardiac cycle and the interpretation of mammalian heart visualisations.
- Plant Physiology - For the last topic in the specification, you'll need to revise the structures/functions of the vascular system within roots, the process of transpiration (including factors that affect the rate of transpiration) and the transport of water through plants.
Bear in mind that this is a simple overview of the areas that you should be revising. The NSAA Specification contains more specific information regarding each topic, so be sure to check it out when preparing your revision.
4/5
HOW SHOULD I REVISE FOR THE NSAA SECTION 2?
By now you should hopefully already be knee-deep in your revision for Section 1, but is there anything different you should be doing to prepare for Section 2? Here are a few tips to help you make the most of your prep time!
1. Choose your Science
Remember, you’ll be answering questions based on a maximum of two of the core sciences throughout both sections of the NSAA. This gives you an important choice to make which will affect how you tackle your revision.
Each section of the exam will require you to answer questions on a single core science, which gives you a few options. A lot of people are likely to choose the same science for each section, which makes sense when you think about it! Specialising in one area will allow you to reduce the amount of revision you need to take on, allowing you to dedicate more time to the area that you’re interested in.
This also helps with focus during the actual exam, as you won’t be shifting between different questions and methods in the middle of the test. After all, why make a hard test even more difficult for yourself?
However, some may welcome the challenge of taking on two different sciences. This certainly isn’t a recommended tactic for everyone, but applicants who are more confident with their knowledge between all three sciences may wish to go this route.
Although specialisation is a good idea for many, that’s not to say that you should neglect the other two parts when revising. You’ll want to make sure you have a general knowledge of all three sciences before going in, as you never know what will happen when you get that paper. You may find that the part you had previously prepared for is more difficult to complete compared to one of the other two.
Exams.Ninja Tip
Before you begin to answer the questions, be sure to briefly read through every question on the paper, not just the ones in the part you’ve chosen. You may find that you are more confident in answering the questions of one part compared to the one that you had initially prepared for.
2. Create a Preperation Plan
This is essential. Having a structure to your revision will help in so many ways and will make the whole process much less painful.
By having a schedule of what you will revise and when, you won’t have to worry about what you’re doing when the time comes to actually revise. That time should be spent revising your knowledge rather than trying to figure out what you need to do, so spending a bit of time before you start to plan everything out will save loads of time in the long run.
Due to the nature of the NSAA, you’ll be able to prioritise certain areas as you have a choice of what you want to be tested on. Of course, you should spend at least some time on each core science, but knowing in advance which of the core sciences you’ll be answering will save you the unnecessary stress of trying to cram everything in at once.
Lastly and perhaps most importantly, having a plan of your revision will allow you to know when you should stop working. Everyone needs downtime when working on a difficult task and revising for an exam is no different. Be sure to schedule regular breaks and days off, or else you’re likely to experience burnout or excessive stress.
You can more more about creating the perfect revision plan with our NSAA 6-Month Preparation Timeline.
3. Practice, Practice, Practice!
There’s no doubt that the best way to learn something is to try it for yourself. Especially with an exam as varied as the NSAA, you’ll need to practice as many types of questions as possible so you can cover all your bases.
Practice questions will be the first thing to look at. Looking at questions with worked solutions is the best way to make sure you understand the answer, there’s no point trying to guess why an answer is correct when you can have it properly explained! Make sure you try a broad range of questions covering all three core sciences so nothing catches you out in the actual exam.
Once you feel comfortable answering questions in this format, it’s time to put your skills to the test with a full exam paper. Whether you tackle just Section 1, Section 2 or both sections together, you’ll benefit greatly by putting yourself in the shoes of someone taking the exam. Recreating realistic exam conditions will help you understand your abilities in an exam environment, including how you perform under time pressure.
Exams.Ninja is a fantastic source for practice questions and past papers for the NSAA. Our NSAA Preparation Platform gives you access to over 1200 practice questions from both sections and 3 full past papers, with worked solutions for everything!
4. Broaden your Horizons
What do we mean by this? Essentially, your revision should go beyond textbooks and practice questions. Section 2 of the NSAA will test you on your ability to take context into account when solving problems. Real-world scenarios are a great way of improving your understanding of context, so try to seek examples wherever you can.
The first thing to try would be news from the scientific community. Whether it’s articles relating to your interests or about something you have no knowledge of, you should be seeking out any kind of additional information that you can to help with your understanding.
You will also find tons of problems and scenarios online provided by actual scientists. The community is extremely collaborative, with people always asking questions to their peers. You can study how these questions have been answered, or even attempt to answer them yourself. If you do this, you’ll likely come into the NSAA and find the questions being asked to be very tame compared to the real-world problems you’ve looked at!
Not sure where to start with your NSAA prep?
Exams.ninja is the perfect place to start your revision, whether you’re looking for guides, practice questions, past papers or everything together! Check out our NSAA Preparation Platform by creating a free account today!
5/5
EXAMPLE PRACTICE QUESTIONS
It’s time for you to try out what you’ve learnt so far with our selection of practice questions. Try them out and see how well you can do, then check out the worked solutions to understand what it all means!
NSAA Advanced Physics Practice Question 1
The equations of a straight line and a circle are given below. Find the points where the 2 lines intersect, (x1, y1) and (x2, y2).
y = 2x + 2
y2 + (x + 1)2 = 125
A) (-5, -8), (5, 12)
B) (-5, -8), (6, 14)
C (-8, -14), (5, 12)
D) (-6, -10), (5, 12)
E) (-8, -14), (6, 14)
F) (-8, -14), (4, 10)
G) (-6, -10), (6, 14)
H) (-6, -10), (4, 10)
The correct answer is H.
Substitute the first equation into the second and solve.
y = 2x + 2
y2 + (x + 1)2 = 125
⇒(2x + 2)2 + (x + 1)2= 125
4x2 + 8x + 4 + x2 +2x + 1 = 125
5x2 + 10x – 120 = 0
x2+ 2x – 24 = 0
(x + 6)(x – 4) = 0
∴ x = -6 or 4
Substitute solutions of x into the first equation for corresponding y values:
x1 = -6 ⇒
y1 = 2 X -6 + 2 = -10
x2 = 4 ⇒
y2 = 2 X -4 + 2 = 10
NSAA Advanced Physics Practice Question 2
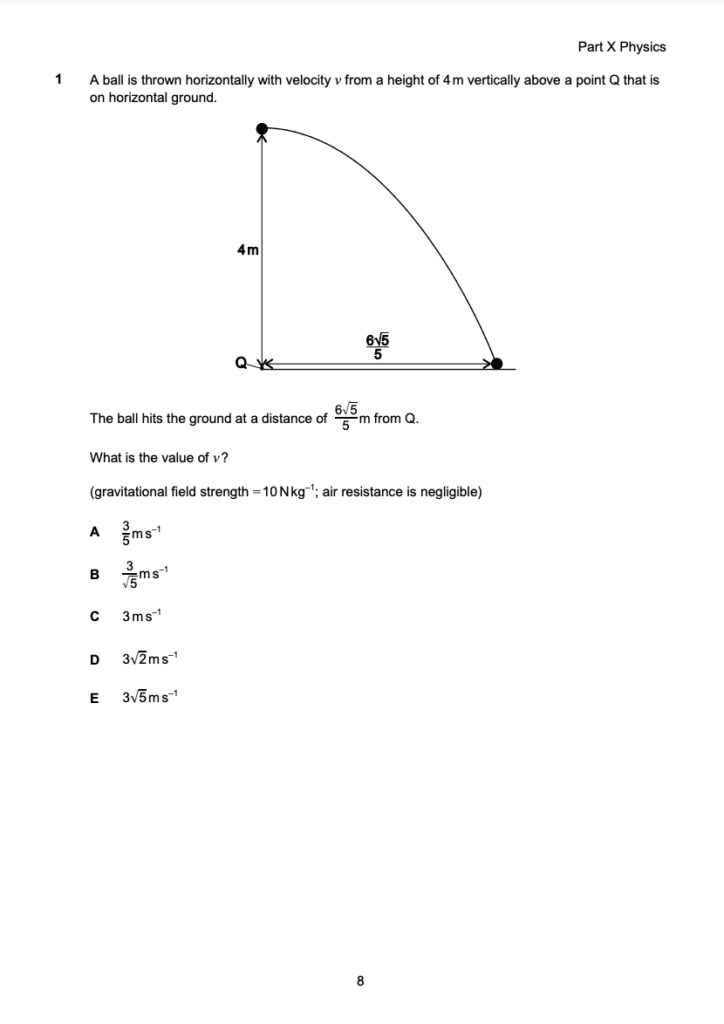
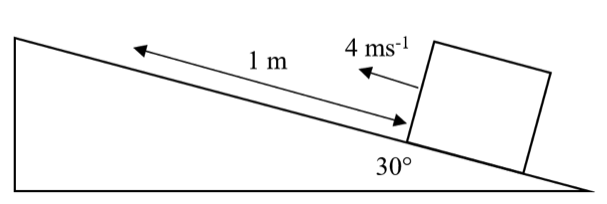
A block of mass 2 kg sliding up a slope has an initial velocity of 4 ms-1. The slope is angled at 30° to the horizontal and the block moves 1 m before it stops. (Take g = 10 N kg-1).
What is the average frictional force on the block as it moves up the slope?
A) 2 N
B) 1 N
C) 3 N
D) 6 N
E) 4 N
F) 5 N
G) 0 N
H) 7 N
The correct answer is D
Do an energy balance for the conversion of kinetic energy into gravitational potential energy and work done against friction.
Change in height:
h = s sin0 = 1sin30 = 10 x 1/2 = 0.5m
Energy Balance:
KE = GPE + WD
1/2 mv2 = mgh + Ffs
1/2 X 2 X 42 = 2 X 10 X 0.5 + Ff X 1
16 = 10 + Ff
Ff = 6 N
NSAA Advanced Physics Practice Question 3
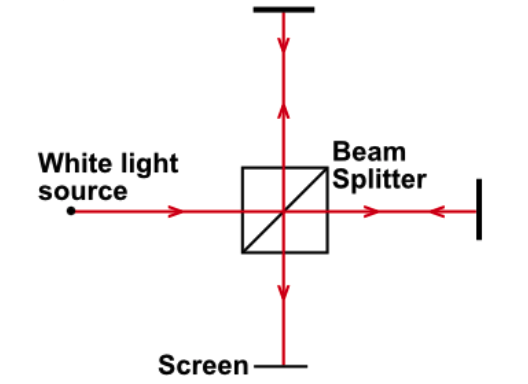
The apparatus shown below is a Michelson Interferometer. A half-silvered mirror (beam splitter) separates the beam into two, which are then directed towards the screen after travelling an equal distance. A bright spot is observed in the centre of the screen.
Now the position of one of the mirrors is varied very slightly, so the distances are no longer equal. As the position of the mirror is varied, the central image on the screen becomes dark and then bright again. The speed of light is 3 X 108ms-1, and the frequency is 6 X 1014Hz. A bright spot is observed on the screen, then the mirror is moved a distance x before it first reappears. How far is x?
A) 1.25 X 10-7m
B) 2.50 X 10-7m
C) 5.00 X 10-7m
D) 7.50 x 10-7m
E) 10.0 x 10-7m
The correct answer is B.
In order for the two separated beams to
constructively interfere and cause a bright spot, the path difference must be an integer multiple of the wavelength (so they arrive in phase). The wavelength is simply found by dividing the speed of light by the frequency (5 X 10-7m). Changing the path difference by a wavelength causes the bright spot to reappear, but any movement of the mirrors has double the effect on the path difference (light travels there and back), so the final answer is half the wavelength 2.5 X 10-7m.
NSAA Advanced Chemistry Practice Question 1
Given the following hydrocarbons: methane (1), ethyne (2), cyclopentadiene (3) and ethene (4). Determine which of the following statements are true:
- The boiling points of these hydrocarbons increase in the order 1 < 4 < 2 < 3.
- The oxidation of (2) with a solution of KMnO4 in H2SO4 gives the same compounds as oxidation of (4).
- The volume of H2 necessary to fully reduce 39g of (2) is 72 dm3 measured at rtp.
- Only (1) can react with Cl2.
- (3) can form 11 cyclic isomers.
A) 1 and 2
B) 2 and 3
C) 1, 2 and 3
D) 2, 3 and 4
E) 2, 3 and 5
F) 1, 2, 3 and 5
G) All of them
H) None of them
The correct answer is F
Statement 1: The boiling point is the temperature when all (or more than 90% of) intramolecular forces are broken and the compound goes from liquid state to gaseous state. For hydrocarbons, the larger the molar mass, the more intramolecular van der Waals forces between the molecules in a sample. Therefore, hydrocarbons with 5 C atoms (3) will have a larger b.p. than those with 2 C atoms (2 and 4) and those with only 1 C atom (1, the only hydrocarbon with 1 C atom). Ethyne has a smaller mass than 2, but due to it being more polar (due to the triple bond), there are more and stronger dipole-dipole forces as well, which compensate for the less van der Waals bonding. Therefore, the order is 1<4<2<3. Statement 1 is correct.
Statement 2: The oxidation equations with KMnO4 in acidic solution are:
HCCH+ 2KMnO4 + 3H2SO4 2CO2 + 4H2O + 2MnSO4 + K2SO4
5H2C=CH2+ 12KMnO4 + 18H2SO4 10CO2 + 28H2O + 12MnSO4 + 6K2SO4
Therefore, the same compounds are obtained. Statement 2 is correct.
Statement 3: n = 39/26 = 1.5 moles; we need 3 moles of H2. Using the ideal gas law, 3 moles of gas occupies 3 X 24 = 72 dm3 at rtp. Statement 3 is correct.
Statement 4: Any multiple bond reacts with Cl2, (2), (3) and (4) will add Cl2 to the double/ triple bonds; (1) can react if first irradiated with UV light. Statement 4 is correct.
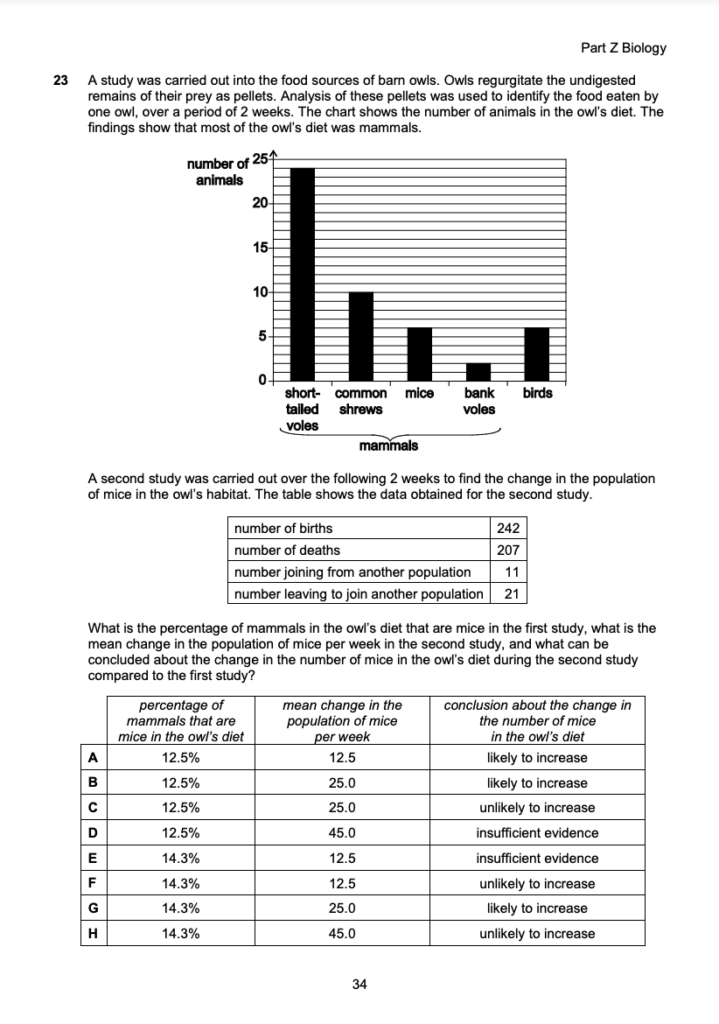
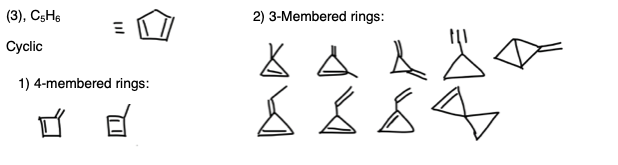
Statement 5: You need to consider 3 and 4-membered rings, as well as the position of the double bond (see diagram below). Notice that the cis/trans isomerism doesn’t apply here. Statement 5 is correct.
NSAA Advanced Chemistry Practice Question 2
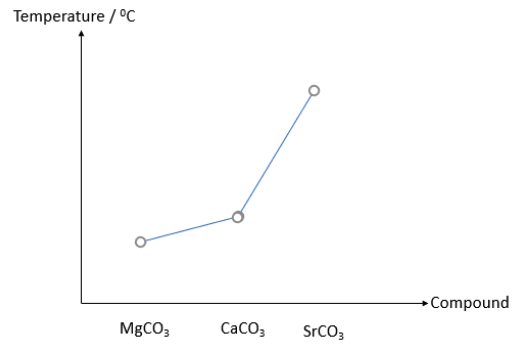
The graph below shows the decomposition temperature for the following Group II carbonates.
Which of the following statements explains why MgCO3 is the least thermally stable?
- CO32- is a large and polarizable anion
- Mg2+ has the lowest charge density
- Sr2+ has the lowest charge density
- Mg2+ polarises the CO32- ion the best
A) none of the 4 statements
B) 1 only
C) 2 only
D) 3 only
E) 4 only
F) 1 and 3 only
G) 2 and 4 only
H) None of the above options
The correct answer is E
All 3 compounds have the same CO32- anion. Statement 1 does not help explain.
All 3 cations have the same charge of +2, but Mg2+ has the smallest radius. Mg2+ has the highest charge density. Statement 2 is false.
Sr2+ indeed has the lowest charge density. However, the question is asking about the thermal stability of MgCO3. Statement 3 does not help explain.
Mg2+ has the highest charge density and thus the greatest polarizing power, causing MgCO3 to decompose the easiest. Statement 4 helps explain.
NSAA Advanced Chemistry Practice Question 3
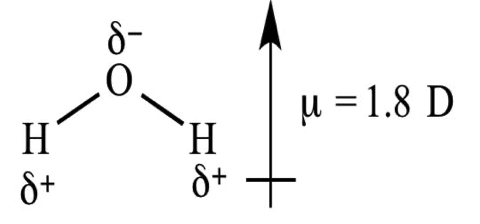
The dipole moment measures the electrical polarity of a molecule due to the different electronegativities of the constituent atoms and is represented by a vector. For example, the dipole moment of water is represented below:
As it is a vector, some molecules can have constituent parts which are very polarized, but their corresponding dipole moments would cancel out.
Which of the following compounds have a dipole moment significantly different from zero?
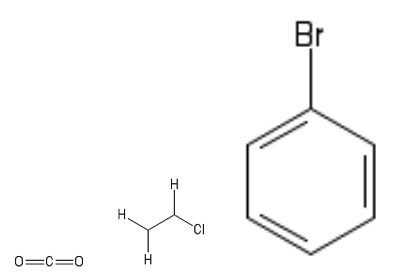
6. The gaseous product obtained when concentrated sulfuric acid reacts with .
7. The compound obtained by monochlorination of ethane.
8. Bromobenzene.
A) 1 and 2
B) 2 and 3
C) 4, 5, 6 and 8
D) 1, 5 and 8
E) 1, 7 and 8
F) 3 and 7
The correct answer is E.
Molecule 6 is CO2 (Acid + Carbonate gives Salt, Water and Carbon dioxide). Molecule 7 is chloroethane. The structures for molecules 6-8 are given below.
Molecules 2, 3, 4, 5 and 6 are symmetrical. Polar dipoles will thus cancel one another out.
Molecule 1 appears symmetrical, but is in fact not, since both polar C – OH dipoles are headed out of the page, yielding a net dipole out of the page.
Molecules 7 and 8 are not symmetrical. The net dipoles arise from the C – CI bond and C-Br bond, respectively.
NSAA Advanced Biology Practice Question 1
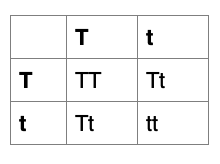
Height in pea plants is controlled by a single gene, with tall and short alleles. The tall phenotype is dominant. A pea plant homozygous for the tall allele is bred with a short plant. The F1 offspring are bred with one another to form the F2 generation. Roughly what proportion of tall and short plants would you expect in the F2?
A) 25% tall, 75% short
B) 75% tall, 25% short
C) 67% tall, 33% short
D) 33% short, 67% tall
E) 100% tall
F) 100% short
G) 50% tall, 50% short
H) Not possible to tell from information given
The correct answer is B.
F0: TT x tt Since tall is dominant, the short plant must have two copies of the short allele
F1: Tt x Tt
F2: TT (25%), Tt (50%) tt (25%)
Since tall is dominant, TT and Tt plants will both be tall. Altogether they make up 75% of the population.
NSAA Advanced Biology Practice Question 2
A freshwater food chain is depicted below
Phytoplankton → Zooplankton → Water fleas →
Stickleback
The primary producer was estimated to contain 250,000 units of energy.
Water fleas are estimated to contain 3,600 units of energy. If the percentage of energy transferred between each stage of the food chain is estimated to be the same at each stage, how many units of energy will be transferred to stickleback?
A) 355
B) 432
C) 396
D) 260
E) 144
F) 576
G) 360
The correct answer is B.
The primary producer starts the food chain, so phytoplankton contain 250,000 units of energy. There are two energy transfers from phytoplankton to water fleas. Across these two transfers, 3600/250,000 = 0.0144 = 1.44% of the original energy has been transferred. To what percentage of energy is therefore transferred across just one transfer, the square root of 0.0144 must be taken, and this = 0.12 = 12%. Therefore, only 12% of 3,600 will be transferred to stickleback so 3,600 x 0.12 = 432 units of energy will be transferred to stickleback.
NSAA Advanced Biology Practice Question 2
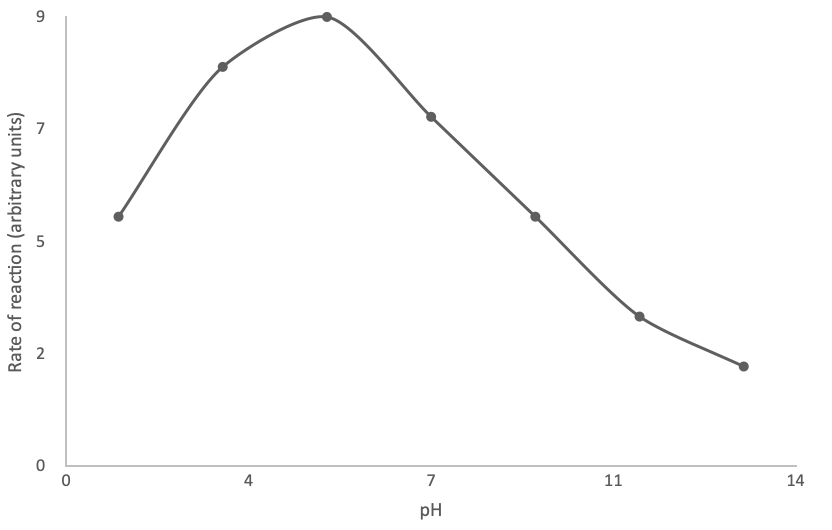
The graph below shows the rate of reaction of an enzyme-catalysed reaction at different pHs.
What is the optimum condition for this enzyme?
A) Very alkaline
B) Slightly alkaline
C) Neutral
D) Slightly acidic
E) Very acidic
F) Neutral and acidic
G) Neutral and alkaline
H) pH independent
The correct answer is D
The optimum pH of the enzyme is when the rate of reaction is fastest. According to the graph, this is about pH 5. This corresponds to slightly acidic.
So, this is what you can expect to be facing in each part of Section 2. Remember, you’ll only be answering one of the three sciences!
So now you’ve gone through everything you’ll need to know, as well as tried a few practice questions, it’s your time to kick your preparation into fifth gear! Use the information you’ve learned today to help create an effective revision plan and start practising everything that you can. Once you start, you’ll find it hard to stop and you’ll be on your way to becoming an NSAA pro!
Exams.Ninja gives you everything you need for your NSAA revision!
When you sign up to the NSAA Preparation Platform, you’ll get instant access to tons of amazing resources to guide you through the NSAA. These include:
Training Temple- Here you’ll find tips, revision notes and over 100 tutorials delivered by NSAA experts.
Practice Dojo- A huge collection of 1200 practice questions covering both sections of the exam. Each one even has a fully worked solution to make sure there’s no confusion.
Exam Arena- Test your skills with our 3 official NSAA past papers. You’ll take each paper with realistic exam restrictions, you’ll get an accurate mark at the end of your test and you’ll be able to see worked solutions for every question.
Get your NSAA preparation off on the right foot today!